Background:
December 1, 2022 is the 35th World AIDS Day.
In July 2022, the latest data from UNAIDS, 2022 Global AIDS Progress Report: Critical Joints showed that the progress in responding to the AIDS pandemic has stagnated in the past two years, 650,000 people worldwide still died of AIDS-related diseases (an average of one death per minute), about 1.5 million new HIV infections (1 million more cases than the global target), and the global AIDS prevention and control situation remains sever.
What is HIV?
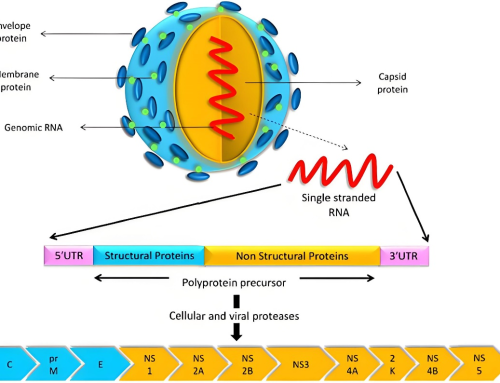
Human immunodeficiency virus (HIV) is a sexually transmitted lentivirus that can lead to acquired immunodeficiency syndrome (AIDS), a condition that leads to gradual failure of the immune system. From the clinical indicators, CD4 + T cells in human blood above 200 are HIV infected, and below 200 are directly judged as AIDS patients.
There are two major types of HIV, type 1 (HIV-I) and type 2 (HIV-II). HIV-I viruses are further divided into M, N, O, and P. M viruses are the most common class and are the main cause of the AIDS pandemic. Class O viruses, “O” represents “outliers”.
HIV has three routes of transmission, sexual transmission, blood transmission and mother-to-child transmission. Among the sexual transmission routes, HIV transmission through gay sex is more likely.
There is no effective AIDS vaccine. Although existing antiviral drugs can suppress the virus and effectively delay disease progression, drugs that can completely cure AIDS have not yet been available.
Diagnosis:
Laboratory diagnosis is the only way to confirm HIV infection, and specific serological markers can be detected early in the course of infection:
HIV RNA: detected by molecular methods, 11 days after HIV infection
HIV-I P24 antigen: detectable 16 days post infection
HIV antibody: detected within 22 days of infection.
In the early stages of infection (acute retrovirus syndrome), flue-like symptoms are accompanied by sudden replication of the virus, which can be detected in the blood. The detection of P24 antigen (viral capsid protein) is directly related to the number of viruses circulating (viral load) in infected individuals.
Antibodies against specific HIV proteins and glycoproteins (e.g., p24, gp41, gp120) are produced 2-8 weeks after infection and are detectable in blood thereafter.
The screening test most widely used to detect HIV exposure is the “HIV antibody test”. The first test was approved by the FDA in 1985 and remains one of the WHO recommended HIV diagnostic methods. Advances in technology and critical reagents have enabled the development of next-generation HIV antibody tests to enable earlier and more accurate detection of infected individuals. The fourth-generation HIV antibody test is able to diagnose HIV infection 3-4 weeks after transmission by detecting both HIV antibody and p24 antibody.
What can Bio-Mapper Provide?
The Maiyue Bio-Mapper technology team has devoted itself to HIV antigen & antibody research and development for many years and successfully marketed a series of products. In addition to providing raw materials with blood as the test sample and applicable to Immunochromatography/Flurescence Chromatography platform, Bio-Mapper has the ability to provide antigen/antibody raw materials applicable to ELISA/Plate Luminescence platform, even Tubular Magnetic Particle Chemiluminescence platform. Maiyue Bio-Mapper’s product line is extremely rich.
There are three routes of transmission of HIV , and HIV, the HIV antigen, is seen in large amounts in semen, vaginal secretions, preseminal fluid, rectal fluid, blood, and breast milk. However, HIV virus is not present in the urine of HIV-infected individuals, and extremely trace amounts of HIV virus may be present in saliva, extremely trace amounts so that infection caanot be caused.
Although HIV antigens are absent or present in very small amounts in urine and saliva, certain amounts of HIV antibodies can be detected in both urine and saliva of HIV-infected individuals.
The recombinant antigen provided by Maiyue Bio-Mapper can be used to detect HIV antibodies in urine and saliva. Among these, gp41 site recognizes binding HIV-1 antibodies and gp36 is used to recognize novel antibodies that bind HIV-2. The epoch-making HIV urine test and saliva test products help health care institutions to carry out HIV preliminary screening more quickly and efficiently. Because the person to be tested can collect saliva and urine samples by themselves, the product can also be applied to personal home self-testing, greatly improving convenience. At the same time, because the test is non-invasive and bloodless (the amount of HIV in the blood is large and the risk of transmitting AIIDS is extremely high), there will be no problem of “infection”, and the risk of infection of the tester or medical staff, the risk of occupational exposure of the sampling personnel, and the risk of infection of medical waste can also be avoided.
Conclusion:
Anti-epidemic, anti-AIDS more than. Maiyue Bio-Mapper’s HIV testing raw materials products will be able to contribute a small force to the global AIDS control cause!
Reference:2022 Global AIDS Progress Report: Critical Joints