The World Health Organization (WHO) estimates that approximately 71 million people worldwide are infected with the Hepatitis C virus (HCV), with a significant portion of them not receiving timely diagnosis and treatment. HCV infection can lead to diseases such as cirrhosis and liver cancer, posing a significant threat to human health. Currently, the primary method for HCV detection is through blood tests conducted in hospitals or clinics, but this approach is plagued by high costs, long processing times, and delayed results. Therefore, rapid diagnostic testing for HCV holds crucial importance as a swift, convenient, and privacy-protecting method of detection.

Introduction to Rapid HCV Diagnosis
What is the HCV Antibody Rapid Diagnostic Kit?
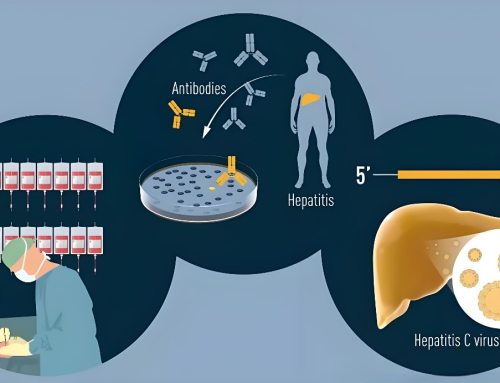
The HCV Antibody Rapid Diagnostic Kit is a testing reagent used to determine the presence of Hepatitis C virus (HCV) antibodies in human serum. This kit employs a rapid immunochromatographic assay, allowing for sample testing within 15-20 minutes. It represents a rapid, accurate, convenient, and cost-effective method for HCV detection.
Technical Principles and Advantages of HCV Antibody Rapid Test Kit
The HCV Antibody Rapid Diagnostic Kit employs an immunochromatographic assay that leverages the specific reaction between HCV antigens and antibodies. This facilitates the binding of HCV antibodies from the sample to the detection line and control line within the kit, enabling the detection of HCV antibodies. This method possesses the following advantages:
- Rapid: The testing process is quick, with results typically obtained within 15-20 minutes.
- Accurate: Results are precise and reliable, comparable to traditional serological testing methods.
- Convenient: The procedure is simple and doesn’t require specialized skills or equipment, making it suitable for on-site testing and remote areas.
- Economical: The reagent kit is cost-effective, suitable for large-scale screening and monitoring.
Working Principle and Usage Process of the HCV Rapid Diagnostic Kit
The HCV rapid diagnostic kit typically consists of components such as a test card, sample collector, buffer solution, and an instruction manual. The steps for conducting HCV antibody testing using this kit are as follows:
- Use the sample collector to collect capillary blood from a fingertip or venous blood.
- Place a drop of the collected blood into the sample well of the test card.
- Add an appropriate amount of buffer solution and wait for 15-20 minutes.
- Observe the test results to determine the presence of HCV antibodies.
Prospects of HCV Testing Market
As people’s awareness of health continues to increase, the HCV testing market is gradually expanding. According to the latest data from Allied Market Research in September 2022, the global HCV testing market was valued at $1.3 billion in 2021, and it is projected to reach $2.3 billion by 2031, with a compound annual growth rate (CAGR) of 5.3% from 2022 to 2031.

Additionally, according to customs data, the import transaction value of HCV rapid diagnosis products has shown a year-on-year increase from 2021 to 2023. It is projected to reach $78,890,645.47 by the end of 2023. This expansion indicates a further growth in the size of the HCV testing market.
Further Increase in Demand for Hepatitis C Testing Market
In May 2022, the 75th World Health Assembly adopted the “Global Health Sector Strategy on HIV, Viral Hepatitis, and Sexually Transmitted Infections 2022-2030” (referred to as the “2022-2030 Strategy”). Within this strategy, the Hepatitis C targets are outlined as follows:
- New infections to decrease from 1.575 million cases (20/100,000) in 2020 to 1 million cases (13/100,000) by 2025, and further to 350,000 cases (5/100,000) by 2030.
- CHC (Chronic Hepatitis C) deaths to decrease from 290,000 cases (5/100,000) in 2020 to 240,000 cases (3/100,000) by 2025, and further to 140,000 cases (2/100,000) by 2030.
These targets will further elevate the demand for the HCV rapid testing market.

Product Recommendations
HCV diagnosis is currently primarily focused on molecular diagnostics and rapid diagnostics. Among them, rapid diagnostic products such as colloidal gold immunochromatography are mainly used for clinical screening. They are characterized by their quick response, ease of use, and cost-effectiveness. Currently, our company has independently developed the following HCV-related products: