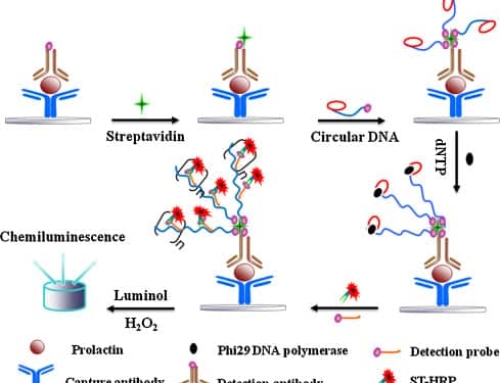
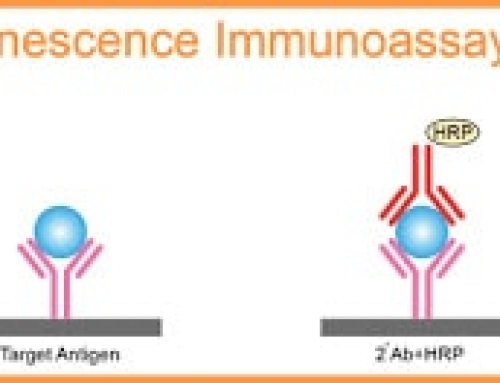
In 1971 Engvall and Perlmann published an article on enzyme-linked immunosorbent assay (enzymelinkedimmunosorbentassay, ELISA) for the quantitative determination of IgG, which led to the development in 1966 of an enzyme-labeled antibody technique for antigen localization into the determination of trace substances in liquid specimens.
I. Principles of ELISA
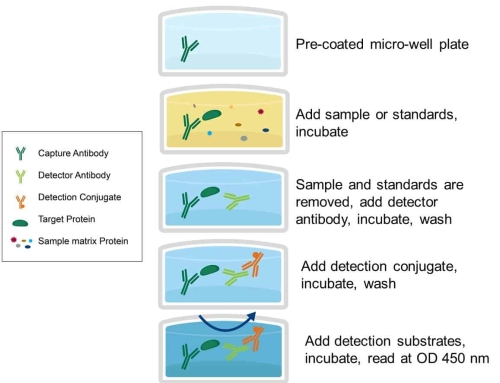
ELISA is based on the solidification of antigens or antibodies and the enzyme labeling of antigens or antibodies. Antigens or antibodies that bind to the surface of the solid carrier remain immunoreactive, enzyme-labeled antigens or antibodies retain both their immunological activity and enzyme activity.
When tested, the substance (antigen or antibody) in the sample binds to a fixed antibody or antigen. The amount of enzyme that can be immobilized is correlated with the amount of substance tested in the sample. The content of the substance in the sample can be judged according to the depth of the color by adding the substrate to react with the enzyme, and the qualitative or quantitative analysis can be carried out.
because of the high catalytic efficiency of the enzyme, the results of the immune response are indirectly amplified, which makes the determination method achieve high sensitivity.
II Basic types of ELISA
ELISA can be used to determine antigens or antibodies. There are three necessary reagents in this method:
1. A solid antigen or antibody;
2. Enzyme-labeled antigens or antibodies;
3. A substrate for enzymatic action.
Different kinds of detection methods can be designed according to the source of the reagent and the characteristics of the specimen and the conditions for the detection.
1. Double antibody sandwich method for antigen
monoclonal antibodies against two different antigenic determinants on antigen molecules are used as solid-phase antibodies and enzyme-labeled antibodies, respectively. Applicable to Determination II Large molecular antigens above valence or divalent are not suitable for the determination of semi-antigens and small molecular monovalent antigens because they cannot form a two-point sandwich.
2. Competition method for antigens
First, the specific antibody was coated on the surface of the solid carrier and divided into two groups after washing: one group of mixed solution with enzyme labeled antigen and the tested antigen, the other group with only enzyme labeled antigen, and the color of the substrate after incubation washing, the difference of the degradation amount of the two groups of substrates, that is, the amount of unknown antigen to be determined. This method is commonly used for the determination of small molecular antigens such as hormones and drugs whenever there is a binding site. Advantages is fast, the drawback is the need for more amounts of enzyme-labeled antigens.
3. Immunosuppressive method for antigen
The inhibition of substrate color is proportional to the amount of antigen contained in the sample, and the difference between them is the amount of antigen predicted.
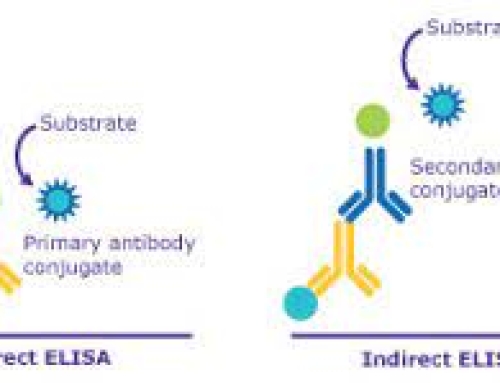
4. Indirect antibodies
It is the most commonly used method to detect antibodies. The principle is to use enzyme-labeled antibodies to detect the tested antibodies that have been combined with solid phase.
All kinds of antibodies corresponding to antigens can be detected with one enzyme-labeled antibody. Mainly used for the detection of pathogens for infectious diseases Diagnosis.
III. Operational steps
Method I Biantibody sandwich method for detection of unknown antigen:
1. Wrap: Use 0.05 MPH9. the sbc was diluted to a protein content of 1 to 10μg/ml by the sbc. add 0.1 ml to the reaction holes of each polystyrene plate at 4°c overnight. The next day, the solution in the hole was discarded and washed with washing buffer for 3 minutes each time. Wash, for short.
Add a certain dilution of the sample 0.1 ml in the above coated reaction hole, incubated at 37°C for 1 hour. And then Washing. (Make blank holes, negative control holes and positive control holes).
3. Add enzyme-labeled antibody: add 0.1 ml of freshly diluted enzyme-labeled antibody (dilution after titration) to each reaction hole. incubate at 37°C for 0.5 to 1 hour and wash.
4. Color rendering with substrate solution: add 0.1 ml of temporarily prepared TMB substrate solution to each reaction hole at 37°C for 10~30 min.
5. Termination reaction: Add 2M sulfuric acid 0.05ml to each reaction hole.
6. The results can be judged by using the naked eye directly on a white background The darker the color in the hole, the stronger the positive degree. The negative reaction is colorless or extremely light. O · D value can also be measured: on the ELISA tester, at 450 nm (if ABTS color,410 nm), the O · D value of each hole is measured after adjusting zero with the blank control hole, and if it is more than 2.1 times of the OD value of the negative control, it is positive.
Method 2 Indirect method for detection of unknown antibodies:
the known antigens were diluted to 1 to 10μg/ml with a coating buffer of 0.1 ml per pore at 4°c overnight. Washing the next day Three times. Add a certain dilution of the sample (unknown antibody)0.1 ml in the above coated reaction hole, incubated at 37°C for 1 hour, washing. Add 0.1 ml of freshly diluted enzyme-labeled second antibody (anti-antibody) to the reaction hole, incubate at 37°C for 30-60 minutes, wash and wash with DDW for the last time. The remaining steps are the same as 4,5,6 of the “double antibody sandwich method “.
IV. Points for attention
1. In the formal test, the test conditions should be controlled by positive control and negative control respectively, and the samples to be tested should be made in duplicate to ensure that accuracy of the experimental results. Sometimes the background is higher, indicating that there is a non-specific reaction, can be closed with sheep serum, rabbit serum or BSA.
2. In ELISA, the selection of experimental conditions is important, including:
(1) Selection of solid supports: Many substances can be used as solid supports, such as PVC, polystyrene, polyacrylamide and cellulose. Its form can be concave plate, test tube, bead, etc. At present, the commonly used hole plate is 40-hole polystyrene concave plate. No matter what kind of carrier, can be screened before use: with the same amount of antigen package, under the same experimental conditions The results showed that the adsorption performance was good.
(2) Selection of coated antibody (or antigen): When adsorbing antibody (or antigen) on the surface of solid carrier, the purity should be good, and the PH should generally be between 9.0 and 9.6 when adsorbing. adsorption temperature, time and its protein amount also have certain effects, generally using 4°C for 18 to 24 hours. The optimum concentration of the protein coating needs to be titrated, i.e. after the coating was carried out with different protein concentrations (0.1,1.0 and 10μg/ml, etc.), the positive specimen was observed when the other test conditions were the same OD value. Select the concentration with the largest OD and the least protein. for most proteins is usually 1 to 10μg/ml.
(3) Selection of the working concentration of the enzyme-labeled antibody: first, titration of the initial titer using direct ELISA (see enzyme-labeled antibody portion). The other conditions were then fixed or the “square method “(the inclusions, the reference of the sample to be examined and the enzyme-labeled antibodies were different dilutions) were accurately titrated in the formal experimental system.
(4) Selection of substrate and hydrogen donor of the enzyme: the choice of hydrogen donor is cheap, safe and obvious color reaction, while itself colorless. Some hydrogen donors (such as OPD, etc.) have potential carcinogenic effects and should pay attention to protection. Conditional people should use non carcinogenic, high sensitivity hydrogen donors, such as TMB and ABTS are currently more satisfactory hydrogen donors. after a period of substrate action, strong acid or strong base should be added to terminate the reaction. usually substrate action time, to 10-30 minutes is appropriate. substrate use solution must be freshly prepared, especially H202 before use.